Pharmacy Safety Standards, Hazardous Drugs and Mechanical Design: What You Need to Know
More than 8 million U.S. healthcare workers are potentially exposed to hazardous drugs in the workplace each year, according to the Centers for Disease Control and Prevention.
Exposure can occur during any aspect of handling hazardous drugs, such as transport, storage, compounding, disposal or even treating patients.
As a result, sweeping changes are occurring within the industry including major changes in the design, construction and operation of pharmacies.
New standards outlined under U.S. Pharmacopeia General Chapter 800 (USP 800) and enforced by the Food and Drug Administration (FDA) and state pharmacy boards were enacted Dec. 1, 2019. These code changes were created to promote patient safety and protect healthcare personnel who handle hazardous drugs (HDs) known to cause infertility, miscarriages, genotoxicity, leukemia and other forms of cancer. The National Institute for Occupational Safety and Health (NIOSH) maintains a list of HDs used in healthcare.
USP 800 establishes design guidelines for the creation of a sterile environment to prevent unintended physical, chemical and microbial contamination of drugs while improving quality and variability in intended strengths.
Facility and engineering controls are summarized in the sections below with the purpose of establishing contamination control areas for operational practices for the receipt, storage, handling and compounding of HDs.
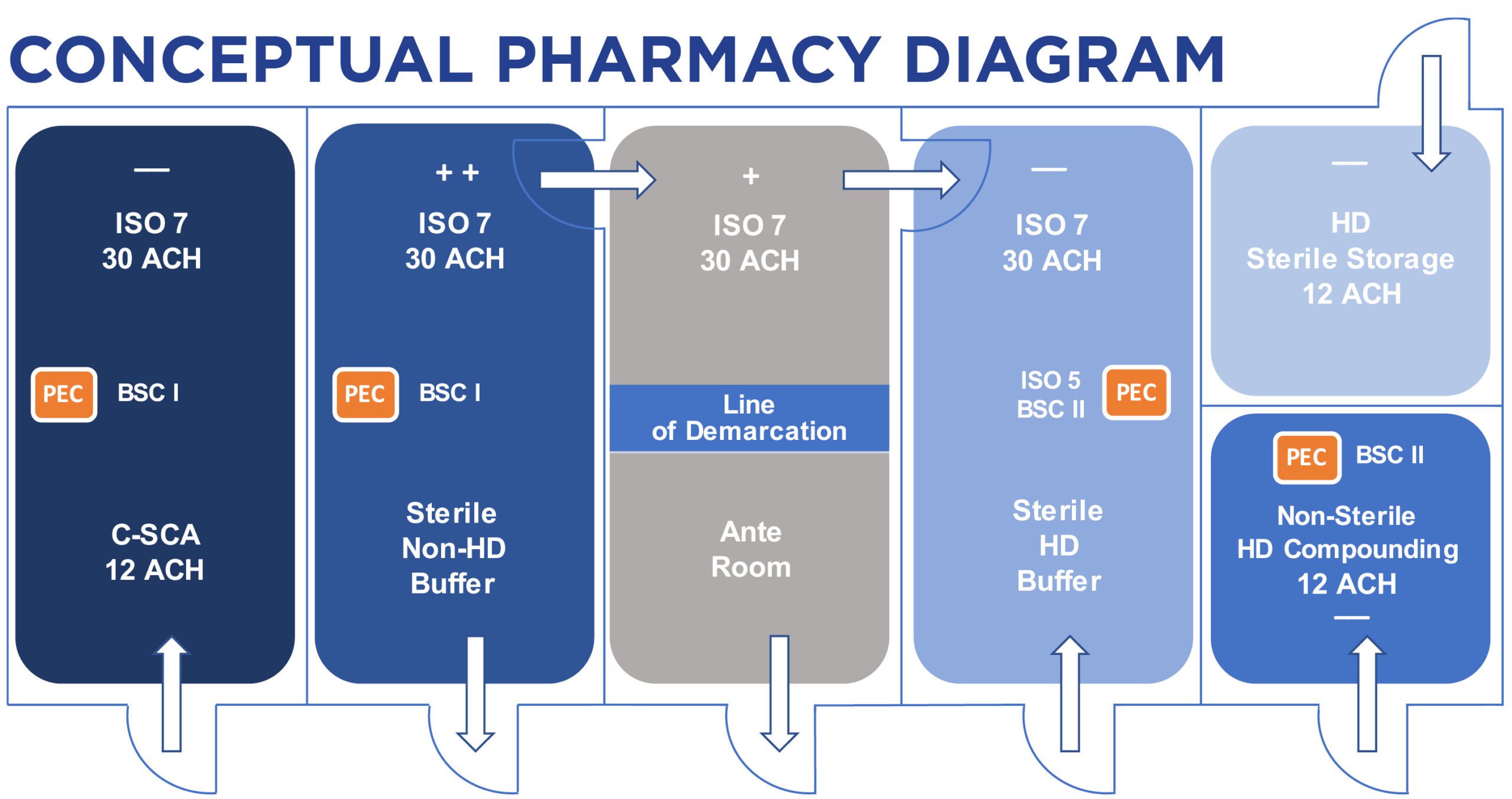
This is one possible layout within the new standards. Many other design considerations can come into play.
Pharmacy Layout (jump to section)
ANTE ROOM
The ante room is adjacent to the clean room, where technicians perform support tasks and are equipped with items such as a sink, cabinets and a bench. It is similar to a waiting room. A line of demarcation is identified, which is not an actual physical separation in the room, but designates an area for personnel gowning and wash.
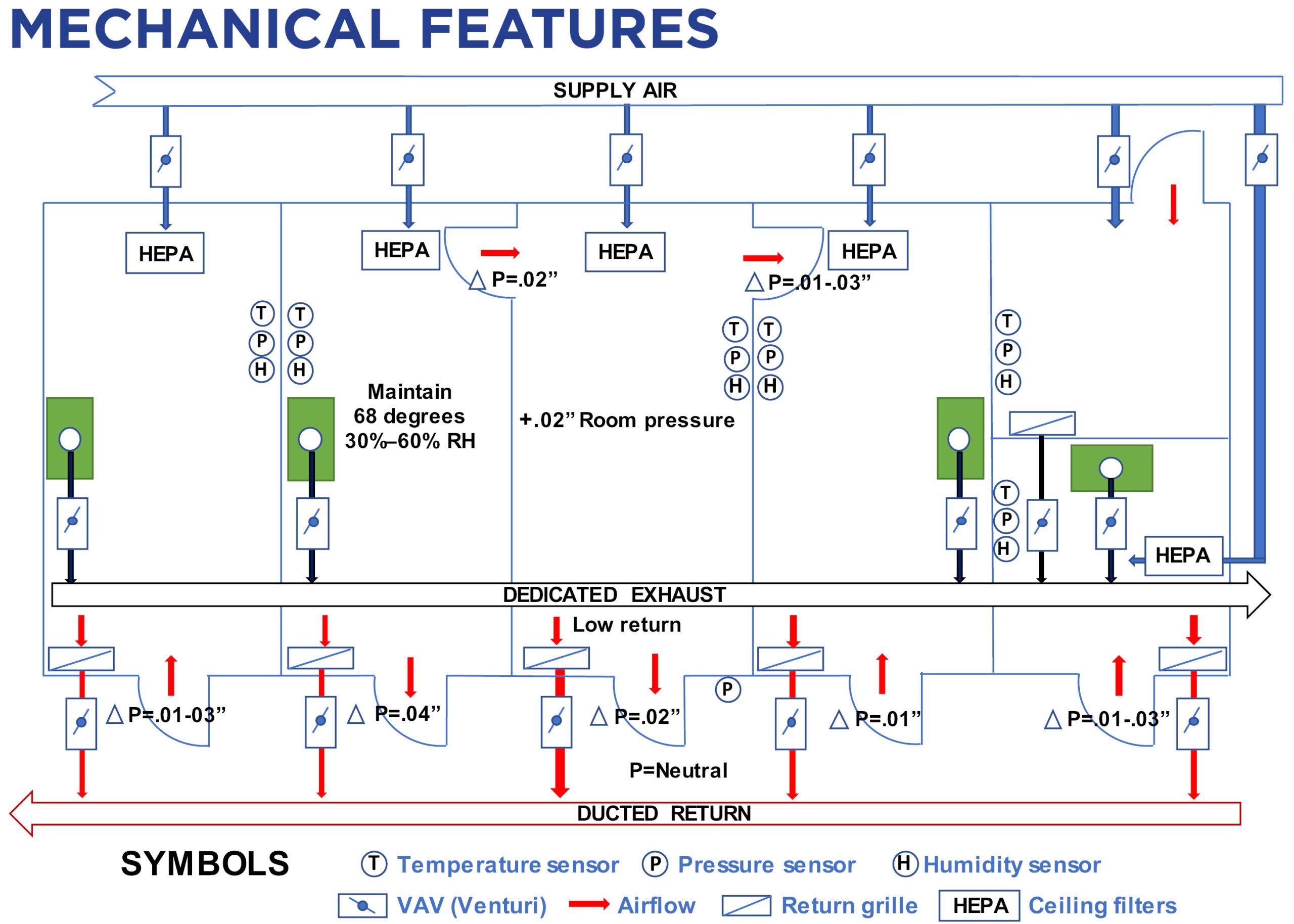
Airflow rates in the amount of 30 air changes per hour (ACH) with laminar airflow distribution are required with HEPA filters to capture pInsights that may promote microbial growth. The environmental condition must meet ISO Class 7 air quality. (The ISO classification system identifies ISO 1 as being the cleanest air, while ISO 9 is the dirtiest. ISO 7 is a common class.)
The room pressure is to be maintained at a positive pressure relative to the sterile buffer room. The room temperature must be designed to maintain 68 degrees Fahrenheight with relative humidity between 30 and 60 percent. A hands-free sink must be available for handwashing, and an eyewash station and/or other emergency or safety precautions must be established. Water sources and drains must be located at least 1 meter away from the sterile HD buffer room.
STERILE HD BUFFER
The HD buffer room is the clean room where the containment primary engineering control (C-PEC), or hood, is physically located. Activities that occur in this area include the preparation and compounding of HDs. The room itself is referred to as the containment secondary engineering control (C-SEC) because it contains the hood used for handling and compounding of HDs. The hood is an ISO Class 5 laminar flow device. These are usually biological safety cabinets (BSC) Class II.
A compounding aseptic containment isolator (CACI), normally referred to as a glove box, can also be used for the compounding of sterile hazardous drugs. These PECs incorporate HEPA filters and are exhausted outside the building with an upblast fan for vertical discharge on the roof. A CACI is used for compounding HDs only and shouldn’t be confused with a compounding aseptic isolator (CAI), which is used for nonhazardous drugs.
Like the ante room, 30 ACH of HEPA-filtered air is required to attain the ISO Class 7 air quality. The buffer room must be at negative pressure relative to the ante room. The temperature and humidity must be maintained at 68 degrees Fahrenheight and between 30 and 60 percent, respectively.
A pharmacist draws fluid from a vial in a laminar airflow hood.
NON-STERILE COMPOUNDING
This is the room where non-sterile HD compounding is performed. The preparation is performed in a primary engineering control that provides personnel and environmental protection, such as a Class I BSC or a containment ventilated enclosure (CVE), and is vented externally. The room must have at least 12 ACH and be maintained at a negative pressure between 0.01 and 0.03 inches of water column relative to adjacent areas.
STORAGE OF HDS AND NON-HDS
The storage of HDs should have at least 12 ACH and be at negative pressure between 0.01 and 0.03 inches of water column relative to adjacent areas. No HDs are to be stored on the floor, which prevents adequate cleaning of surfaces. Sterile and non-sterile HDs can be stored in the same room if the room is maintained at a negative pressure. Also, there must be a clear separation for the storage of hazardous and non-hazardous drugs. Refrigerated HDs must be stored in a dedicated refrigerator, which must have a dedicated exhaust behind the unit to remove pInsights that may accumulate on the condenser. HDs and non-HDs cannot be stored in the same room.
CONTAINMENT SEGREGATED COMPOUNDING AREA (C-CSA)
The compounding of low- and medium-risk HDs with a beyond-useful life of 12 hours can be performed in a separate room with a Class I BSC. The room must be a negatively pressurized room between 0.01 and 0.03 inches of water column relative to adjacent areas with 12 ACH of HEPA-filtered laminar supply air.
Pharmacies must have pressure- and temperature-monitoring capabilities to become licensed and must be documented daily for compliance as part of the biannual inspection process by the state pharmacy boards. This will require pressure gauges positioned where cascading pressure differentials are required; air-measuring devices in the supply, return and exhaust ductwork to regulate airflows at each room; and HEPA-filter monitoring. The PECs must operate continuously to maintain sterile environments, and it’s recommended they operate on backup power.
A recommended pharmacy room layout is shown below (click to enlarge):
Evaluation of existing mechanical equipment will be required to determine HVAC system modifications such as increases in ventilation rates; controls for temperature, humidity and pressurization; and filtration.
Our healthcare engineers can review your existing facilities and make recommendations to ensure compliance.
Contact our MEP Engineering leaders today!